AMT Medical Secures $25 Million in Series B Funding to Revolutionize Coronary Artery Bypass Surgery
/EIN News/ -- Pioneering sutureless coronary bypass technology poised to eliminate open-chest procedures
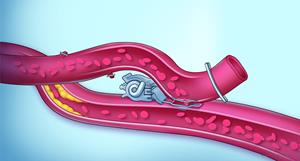
Sutureless anastomosis: Laser-assisted vessel connection via proprietary clip
Beating-heart compatibility: Reduces stroke risks and complications, and potentially over 50% cost reduction with a standardized, reproducible and easy-to-learn technology.
EDE/UTRECHT, The Netherlands – April 17, 2025 – AMT Medical B.V., a clinical-stage medtech innovator, today announced a $25 million Series B financing round led by Bender Analytical Holding B.V. (BAH). New investors Invest-NL, and the European Innovation Council (EIC) joined existing backers Oost NL and informal angels in the round. The funding accelerates the development of AMT’s ELANA® Heart Bypass System, a minimally invasive solution designed to replace traditional open-heart bypass surgery, ultimately leading to robot-assisted keyhole surgery compatible with surgical robots from industry leaders.
The new funding will enable the company to pursue key initiatives, including CE marking and initial clinical trials in the United States in open and robotic settings.
Paradigm Shift in Cardiac Surgery: Key innovations
The ELANA® Heart Bypass System will enable surgeons to perform the most state-of-the-art coronary artery bypass grafting (CABG) with arterial (instead of venous) bypass grafts and through small chest incisions without stopping the heart or using a heart-lung machine, which eliminates stroke risks and other complications and reduces postoperative recovery times. This contrasts starkly with conventional methods requiring sternotomy (chest cracking), complex suturing and long recovery times. With these improvements, the system further aims to significantly reduce costs associated with CABG procedures.
The sutureless anastomosis technique utilizes a proprietary clip and an excimer laser to connect blood vessels without manual suturing, enabling precise, rapid, and standardized and reproducible graft integration while minimizing complications.
Additionally, the system achieves over 50% cost reduction when used in a robotic setting. This is driven by shorter operating room times, reduced hospital stays (patients can return home within days), fewer complications, and faster surgeon mastery compared to traditional coronary artery bypass grafting.
Key initiatives
Following this financing round, the capital will drive AMT’s running initiatives: Completing AMT Medical’s European first-in-human trial evaluating the system during - still - open chest CABG procedures with a beating heart. Results of the trial will be available towards the end of 2025 and presented/published to the surgical community. With this data, AMT foresees obtaining CE Marking by 2026 for use during open surgical CABG procedures, either through sternotomy or via a minimally invasive thoracotomy (MIDCAB). Furthermore, clinical trials will be started in the United States in open and robotic setting.
Rutger Tulleken, CEO & Co-Founder of AMT Medical, commented:
“This financing validates our mission to make open-heart bypass obsolete. By enabling same-day discharge bypass procedures, we’re not just improving outcomes – we’re redefining cardiovascular care.”
Johan Bender, CEO of Bender Analytical Holding:
“We are proud to support AMT as they work toward FDA clearance to bring their technology to the market in the U.S. This marks a key milestone in improving patient outcome”
About AMT Medical
AMT Medical (est. 2017) is an Ede and Utrecht-based innovator spun out of neurovascular ELANA technology which was earlier brought to the market (CE and FDA) by the AMT team. With 27 employees and ISO 13485-certification, the company holds over 40 patents for its beating-heart surgical platform.
AMT secured $40 million in funding including this Series-B Funding round and several multi-million-euro grants from RvO and the European Innovation Council Accelerator. Collaborations with institutions like St. Antonius Hospital in Nieuwegein, The Netherlands, and Charite in Berlin, Germany, position AMT as a leader in next-generation cardiovascular care with goals of expanding into European and U.S. markets, refining fully endoscopic procedures, and reducing global reliance on open-heart surgeries—potentially benefiting 1 million patients annually.
For more information, visit AMT-Medical’s website, or find us on Linkedin.
About Bender Analytical Holding
BAH is an early-stage VC that supports entrepreneurs driving disruptive innovation across medtech, pharma, biotech and high/deep-tech sectors, with a particular focus on those with international ambitions suitable for Strategic exits. The fund invests in seed capital to help initiate international expansion and continues to support entrepreneurs over the long term through follow-on investments.
For further background info, please contact:
AMT-Medical, Ede/Utrecht, the Netherlands
Rutger Tulleken, CEO
Email: info@amt-medical.nl
LifeSpring Life Sciences Communication, Amsterdam, the Netherlands
Leon Melens
Phone: +31 6 538 16 427
Email: lmelens@lifespring.bio
Attachment

AMT’s ELANA® Heart Bypass System, a minimally invasive solution designed to replace traditional open-heart bypass surgery
The sutureless anastomosis technique utilizes a proprietary clip and an excimer laser to connect blood vessels without manual suturing, enabling precise, rapid, and standardized and reproducible graft integration while minimizing complications.
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release